P.S. DyersLIST no longer exists.
DyersLIST is "an internet mailing list intended for the discussion of technical questions, problems and information related to immersion dyeing and to the surface application of synthetic dyes, textile pigments and related chemicals, to fabric and fiber" (list.emich.edu) owned by East Michigan University.
I was a subscriber of DyersLIST from 2002 until 2012. During this time, web search engines (Google etc.) gradually took over most of the information searching done on Internet, and I started to realize that to the contemporary standards, the way the list archives were accessible was very inefficient: One had to subscribe to the list and to log in to the search system using ones password. In 2012, the list has about 500 subscribers, which is a fraction of the number of people in the world that might reach the information via web searches, was the information available. Therefore, I cannot recommend anyone to contribute to DyersLIST, but instead to write on some other dyeing-related forum that has better search engine visibility.
To make my own postings to DyersLIST visible to search engines, I have attached all of them below.
From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sun Jul 7 09:31:00 2002
Context: What is soda ash, washing soda, and baking soda?
Hello!
This is my first post to the list so I'll introduce myself. I'm a
23-year-old (male, if you can't make that out from my name) biochemistry
student from Oulu/Finland. Last year I had a short trip to California, and
bought a truly colorful tie-dye hippie T-shirt with a gekko figure, from a
woman with her baby on Telegraph street in Berkeley, and have been sorry
ever after that I only bought one piece... So, I started experimenting
tie-dyeing myself, reading up on color theory, even selling a few pieces
to my friends (still have orders waiting!) and just this week received
(together with my mother, who's quite an artistic person) a package of
Procion MX dyes from Quilt&Art/Germany. So everything is looking good!
The actual post:
There has been some confusion what soda ash, washing soda, baking soda,
soda, sodium carbonate, sodium bicarbonate and such are. I had this mess
in my head as well, so I did some research and came up with answers that
I'd now like to share:
There are two different chemical compounds: sodium carbonate (Na2CO3) and
sodium bicarbonate (NaHCO3). The one you want to use to control pH in
dyeing is sodium carbonate, which rises pH of the solution to around 11,
but not much above. Sodium bicarbonate has the same effect, but only gets
pH to around 8, which typically is inadequate. Let's take a closer look at
the two substances:
Sodium bicarbonate
"baking soda"
* white powder
Heating sodium bicarbonate above 50 degrees Celsius decomposes it into
sodium carbonate, water and carbon dioxide gas. Note that this means you
can prepare a sodium carbonate solution by boiling a sodium bicarbonate
solution!
Baking soda is readily available in grocery stores.
Sodium carbonate
"soda ash" or shortly "soda"
* white powder
* dehydrate/anhydrate (water-free) or monohydrate (one water per
sodium carbonate). Not that much difference.
"washing soda"
* transparent crystalline
* decahydrate (10 water molecules per sodium carbonate)
Dissolved in water, the two are exactly the same chemical. But as
solids, washing soda contains much more water and, for example in
preparation of a solution, you need to use it roughly 2.5 times in
weight the amount you would use soda ash. (I myself like to prepare
a saturated stock solution by mixing two volume units of washing soda
with at most one volume unit of water)
Heating washing soda (i tested it in my kitchen electric oven) dries it
into soda ash.
As said, you can use either soda ash or washing soda, whichever you
can find cheaper. Note that if soda ash costs twice as much as the same
weight package of washing soda, it's still cheaper in use. Sodium carbonate
can be available as household washing soda (only buy pure, without
brighteners and such additives), a photographic chemical (sodium
carbonate), pool pH controlling chemical (pH UP for one), or, reportedly
from a clay company.
As a final reminder, remember safety issues when dealing with alkalic
substances such as sodium carbonate. Wear protective goggles and gloves
and avoid breathing the dust.
-olli
From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sun Jul 7 10:59:01 2002
Context: What is salamoniac?
Hmm... I didn't know of any use of it in dyeing. All my experience about the substance (ammonium chloride) is through ingestion. :-) If you wanna know more of that... http://www.everything2.com/index.pl?node_id=505027&lastnode_id=320771 Oh and while on that California trip, I had some Tyrkisk Peber candies with me. I had to eat them myself! :-/ -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Wed Aug 7 13:32:01 2002
Context: It has been claimed that Dylon brand dyes sold in England are Procion MX. Is this correct?
I have a Finnish package of black Dylon washing machine dye on which is printed "C.I. Reactive Black 5". C.I. presumably stands for "color index". There was something on the web of this particular dye: "C.I. Reactive Black 5 is one of the most used reactive dyes for textile finishing. It is a diazo dye [...]" (http://www.iwaponline.com/wst/04405/wst044050295.htm) "C.I. Reactive Black 5 [2,7-naphthalenedisulfonic acid, 4-amino- 5-hydroxy-3,6-bis((4-((2-(sulfooxy)ethyl)sulfonyl)phenyl)azo)-, tetrasodium salt] (CAS Reg. No. 17095-24-8)." (http://vm.cfsan.fda.gov/~lrd/cf733127.html) Looks like different stuff from Procion MX to me! (but perhaps someone can tell better) -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Wed Aug 7 15:25:01 2002
Context: Dylon Washing Machine dyes
I gave a local store a quick visit and found these texts on miscellaneous Dylon Washing Machine dye packages. Some more info is added as found from the Web. "Reactive Yellow 125" (azo dye) "Reactive Blue 225" Lithiumnatriumhydrogen-4-amino-6-(5-(5-chlor-2,6- difluorpyrimidin-4-ylamino)-2- sulfonatophenylazo)-5-hydroxy-3- (4-(2-(sulfonatooxy)ethylsulfonyl)phenylazo)naphthalin- 2,7- disulfonat "Yellow DO 2286" "Reactive Red MDO 358" (Drimarene Red R-7B, monoazo dye) I think why the above chemicals are mentioned is because, in Finland, there was a fuss on azo dyes (in candies) causing allergy, so it might be required that azo dyes are mentioned in consumer product packaging. I'm sure at least some of the Dylon packages I inspected contained other dyes in addition to the listed azo ones. Not all of them had any notes on the chemicals at all! -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Thu Aug 8 18:45:01 2002
Context: Any information about Dylon Hand dye ingredients, from Finnish packaging?
Yes, I found some today (such the Finnish name of which translates to "Hand"). Again, they seem mixtures of several dyes, some of which are mentioned (must be this azo thing again). Most read simply "contains reactive dyes" (my translation). The explicitly mentioned dyes I spotted in the Hand series were: "Reactive Yellow DO 2286" "C.I. Reactive Black 5" "Reactive Red MDO 358" So at least some of the dye chemicals are exactly the same as in the Dylon Machine series. -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sun Aug 25 04:53:01 2002
Context: Is bleaching Procion MX -dyed cotton possible?
Yes, it might be possible. Though English is not my native tongue, I think discharge means releasing the dye from the fabric. But what really (usually?) happens, is that the dye molecule is modified by the bleach, removing or changing its colorant properties. So depending on the dye and bleach, bleaching might change or remove, or partially remove, the color of the cloth. You should try both oxidizing (chlorine) and reducing (sodium dithionite) bleaching agents, as either probably has at least *some* effect on the dye. You might end up with a completely different tone of color. I had a cloth that had absolutely no green or yellow dye, but after bleaching it had a pale, greenish tone! It would be interesting to have a chart of the bleachabilities of the different MX reactive dyes. I've experimented a bit with bleaching as part of the dyeing process, and the results are sometimes interesting, though not yet consistent. :-) Usually I end up with pale, clearly defined spots on the cloth instead of nice gradients... -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sat Aug 31 03:36:01 2002
Context: Can I measure microwave oven temperature using an electric thermometer?
You do not want to have the microwave on while the thermometer or its probe is in there, as induction from the microwaves can destroy the device. -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Thu Sep 12 03:43:01 2002
Subject: Mercerization
Are blank T-shirts typically mercerized? Or should I try mercerizing at home? :-) -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Wed Sep 18 12:17:00 2002
Context: In Finland, Procion MX (or similar) dyes can be found under brand name Furian.
I'm also from Finland; where did you find Furian dyes from? I ordered my set of Procion MX dyes all the way from Quilt und Art, Germany. -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Wed Sep 18 12:54:02 2002
Subject: Mercerization
On Thu, 12 Sep 2002, Olli Niemitalo wrote: > Are blank T-shirts typically mercerized? > > Or should I try mercerizing at home? :-) OK, I tried it last weekend... I used a 20% NaOH solution for 5 minutes at room temperature to do the mercerization, and citric acid for neutralization. It was much like Doug said, the cotton fabric became *very* stiff while it had NaOH in it. And it shrank much, perhaps 15%. I did this on two t-shirts, the other of which I wrapped tightly around a piece of wood to avoid shrinkage, but in vain as both shirts turned out much the same. After laundrying and drying, the shirts were really wrinkled and had a sort of "cardboard" feeling to them. One of the shirts was already a bit short, and now it is too short to reach my pants! :-) The other is quite fine and actually looks pretty classy after ironing. This was all quite interesting, but not very useful, and hard to do properly at home. If you want to have the fabric tensioned for the luster effect and less shrinkage, you need a large(ish) supporting system and consequently a large tank where to dip the system in. The amount of NaOH (and neutralizer) wasted is huge. Neither would I recommend this to anyone without lab training (I've had lots). -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Fri Sep 20 03:03:01 2002
Context: In Finland, Furian is sold by Seriväri.
Thanks for the good info! Context: Any more info about the German Quilt und Art? You can take a look at their homepage: http://www.quiltundart.de/ We ordered with a credit card by faxing the order and e-mailing the forgotten credit card security number. (I had to order with my mother as I'm a student and don't have a credit card) Alternatively, you can use e-mail or phone. These MX dyes are available: Color Code euro/100 g box ------------------------------------------ Marine blue MX - 4 RD 12,78 Yelloworange MX - 3 R 12,78 Burnt orange MX - GRN 12,78 Pinkred MX - 8 B 12,78 Citrus Yellow MX - 8 G 15,34 Blueviolet MX - 7 RX 15,34 Turquoise MX - G 15,34 Primary blue MX - G 15,34 Dark Blue (cold) MX - 4 GD 15,34 Cotton Black MX - 602 A 17,90 Orange MX - 2 R 15,34 Magentared MX - 5 B 12,78 ------------------------------------------ ...Plus postage, which is around 6 euro. They also have an offer for 8 preselected 100 g boxes for 92.03 euro. -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sat Sep 28 09:51:01 2002
Subject: Sugar and fiber reactive dyes
Do reactive dyes such as the Procion MX dyes react with table sugar, in solution, in a similar way they react with cellulose fibers? It would seem so: I did some experimenting by adding into a solution of Red MX-8B and soda some 1) dextrose, 2) sucrose (table sugar) 3) nothing. After a while I tried dyeing with the solutions and the one with sucrose gave a much more pale result than the others. There wasn't any significant difference between dextrose and water. In another experiment, I rinsed a freshly dyed piece of fabric (which contained some unreacted dye) in a small amount of 1) water and 2) sucrose solution, with a white cotton test swatch. After the initial rinsing, I washed the pieces and compared the swathes. The one from the sugar solution was somewhat whiter overall, though both were slightly pink from the bled dye (Red MX-8B). Perhaps sugar could be used creatively in dyeing, or to prevent bleeding in the rinses (if huge amounts are not required? I used quite much...). -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Tue Dec 24 22:13:01 2002
Subject: Dye Mixer applet
Here is my little X-mas gift for you people... :-) http://www.ee.oulu.fi/~ollinie/dye/dye.html It is a computer program that allows you to try mix different MX dyes. It is still a bit hard to use, but I will keep working on it. -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Thu Dec 26 13:31:18 2002
Context: How can I open Dye Mixer applet using Microsoft Windows?
The Dye Mixer (which, by the way, is now at version 1.0, with slide bars for changing the amounts of dyes) is a "Java applet" program that runs directly from Internet, in the web browser (in your case Internet Explorer). To use it, you need to install "Java plug-in" for the web browser. To find the Java plug-in download page, open the Dye Mixer page http://www.ee.oulu.fi/~ollinie/dye/dye.html and follow the link where it says "Java plug-in" and you will be taken to a Google search page. In your case, the first link will enter the Java plug-in download and install page, where it says "Click to begin". Proceed, and install the plug-in. You need to close and restart Internet Explorer after the installation has completed. Happy mixing! :-) -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Fri Dec 27 18:21:01 2002
Context: Which Java to download for Dye Mixer applet, 1.4.1?
My keyboard got destroyed so i can't do much on computer now... :/ Java plug-in v1.2 or greater, or rferrably the latest, should do. I'll get back to business, when I get a new keyboard.... -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sun Dec 29 18:48:00 2002
Context: Dye Mixer applet
Thank you everybody for the feedback, and your kind support! I'll try to give some answers collectively in this mail. Compatibility problems ---------------------- The Dye Mixer will only work if you have, or have installed, Java Plug-in 1.2 or later. It should be available free of charge. Google (www.google.com) is your friend. Mac OS 9 or ealier won't work. Seems that the only workaround is to install Mac OS X, which has up-to-date Java support. Linux, and Microsoft Windows operating systems should run the Dye Mixer after installing the latest Java Plug-in (may require restarting the browser). In some cases, you may need to go to the web browser security settings to enable Java. Please, if you send a problem report, always specify your operating system and its version, and your web browser and its version. Adding a new dye ---------------- Each dye in the Dye Mixer has been carefully analyzed with a spectrophotometer, and the resulting spectral data are used. To add a new dye, I either need to obtain the measured data or receive a small sample of the dye so that I can do the analysis myself. Saving settings --------------- Currently it is impossible to save the dye recipe by other means than writing it down yourself. This is something I plan to work on to provide a more reasonable solution. In Windows, to take a screen shot, press Print Screen on your keyboard and paste the picture into any image processing program. In reply to other suggestions ----------------------------- I will add a link to Paula's great page. The relative amount values might be confusing, but I really don't know a better way. -olli, from freezing-cold -29C/-20F Oulu, Finland From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sun Jan 5 22:31:00 2003
Context: What do the amount values in the Dye Mixer applet mean? They apparently are not related to the sliders.
Oh but they are! The idea is that you use the slider bars to change those numbers. The numbers range from very small numbers to quite large numbers, so I had to make the sliders non-linear. That is, most of the range of a slider operates on the "small numbers" range, and large numbers can only be adjusted very coarsely using the slider. And what are these numbers? They are values proportional to the amount of dye in the canvas. For example, you try dyeing a piece of cloth using 2 g of actual dye. In dye mixer, you get the same resulting color by adjusting the Dye Amount value to 1.5. However, you want a deeper shade for your cloth, the one that you see by adjusting the Dye Amount value to 3.0. This is a two-fold increase from 1.5, so you also need to increase your 2 g to 4 g, that is: 3.0/1.5 * 2 g = 4 g This is presuming that doubling the amount of dye in your dye bath will also double the amount of dye in the canvas. Perhaps that 2 g is a "per 1 kg of cloth" type of a measure. However great it would be, I do not plan to make it possible to use real-world dye amounts in the Dye Mixer any time soon. Now I am working on a new version which will include some more dyes, lamps and hundreds of different "canvases" (most just to function as color references). And - it will work in most browsers straight-from-the-box without installing Java from Sun. The link, just in case: http://www.ee.oulu.fi/~ollinie/dye/dye.html -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Mon Jan 6 14:25:01 2003
Context: Dye Mixer applet, a confusion on how to calculate real amount of dye.
[...] No, not like that at all. Hmm.. You should perhaps read once more the instructions on the page and my previous mail (in which I was talking of a single dye, not a dye combination). [...] I didn't say such a thing. I'll try to explain again... You should think of the Dye Amount value as being of an unknown unit. I cannot have it in grams or such because the actual amount of dye consumed is different for each combination of dye, dyeing method and type of canvas, and all the other additional parameters. All I am doing is giving you a proportional measure which helps you in the way that you can make observations like: "Oh, I need to double / halve / triple / whatever the amount of dye to move from *this* shade to *that* shade". -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Fri Jan 10 18:41:01 2003
Subject: SDC Online Resource File
For those interested in "who makes the dyes" and such things, but can't afford The Colour Index, there appears to be a nice on-line industry resource: http://www.sdc-digital.org/resource/ -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Wed Feb 26 10:19:01 2003
Context: New subscriber introduction and where to buy Procion MX in EU?
Welcome to the list! I get mine from Quilt & Art, Germany: http://www.quiltundart.de/Deutsch/D_download.html They accept credit cards. I have also paid them with a money transfer which cost 7 euro in a Finnish bank done through their Internet interface. -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sat Mar 29 14:58:00 2003
Subject: "hairy" results in cotton LWI / Procion MX
Hello, list! I have been doing low water immersion dyeing on cotton using Procion MX dyes. I nearly always get results where small white fibres stick out of the otherwise nicely dyed fabric, making the darker colors slightly pale. Only in spots where the fabric was immersed, the problem is absent. Is there a solution to this problem? I am using water + Procion MX + soda ash + (sometimes) salt on machine-washed, dry, unmercerized cotton cloth. -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
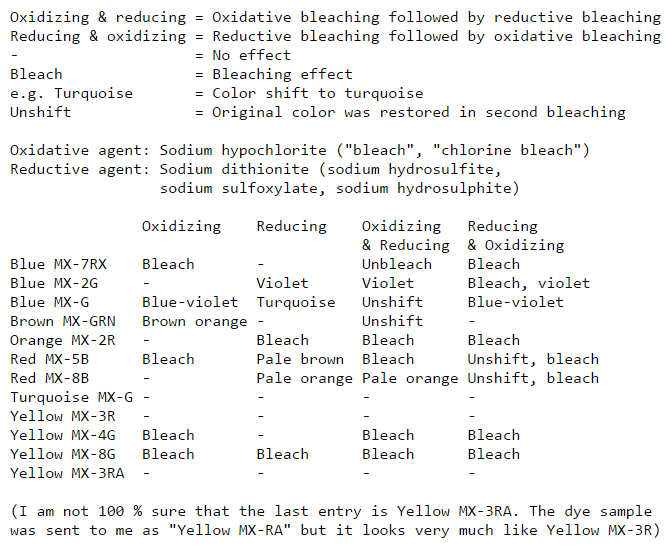
Date: Mon Jun 30 03:19:01 2003
Subject: Procion MX dye bleaching table
Hello!
I have been testing different Procion MX dyes for how they can be bleached
off cotton. Here are the results as a table (best viewed with a monospace
font).
Explanations:
Oxidizing & reducing = Oxidative bleaching followed by reductive bleaching
Reducing & oxidizing = Reductive bleaching followed by oxidative bleaching
- = No effect
Bleach = Bleaching effect
e.g. Turquoise = Color shift to turquoise
Unshift = Original color was restored in second bleaching
Oxidative agent: Sodium hypochlorite ("bleach", "chlorine bleach")
Reductive agent: Sodium dithionite (sodium hydrosulfite,
sodium sulfoxylate, sodium hydrosulphite)
Oxidizing Reducing Oxidizing Reducing
& Reducing & Oxidizing
Blue MX-7RX Bleach - Unbleach Bleach
Blue MX-2G - Violet Violet Bleach, violet
Blue MX-G Blue-violet Turquoise Unshift Blue-violet
Brown MX-GRN Brown orange - Unshift -
Orange MX-2R - Bleach Bleach Bleach
Red MX-5B Bleach Pale brown Bleach Unshift, bleach
Red MX-8B - Pale orange Pale orange Unshift, bleach
Turquoise MX-G - - - -
Yellow MX-3R - - - -
Yellow MX-4G Bleach - Bleach Bleach
Yellow MX-8G Bleach Bleach Bleach Bleach
Yellow MX-3RA - - - -
(I am not 100 % sure that the last entry is Yellow MX-3RA. The dye sample
was sent to me as "Yellow MX-RA" but it looks very much like Yellow MX-3R)
About anti-chlor... I think the reason why it is needed is because in
neutral (and acidic) conditions, such as after the cloth is washed after
bleaching, hypochlorite turns partly into hypochlorous acid which is very
effective in destroying the cellulose fibers. (This information is from
http://www.wmich.edu/ppse/pekarovicova/160999b.html)
-olli
From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Sun Jul 13 16:23:01 2003
Context: Why do wet clothes look darker?
White fibers such as cellulose are actually transparent, but they have a different refractive index than air. Therefore, when a ray of light enters a dry, white fabric, it changes its direction each time it crosses a boundary between fiber and air. This results in the ray bouncing around, until it exits the fabric, and possibly enters one of your eyes. When the fabric is wet, the air inside the fabric is replaced by water. Compared to air, the refractive index of water is much closer to that of the fiber. When the difference in the refractive indexes is smaller, the light does not change its direction so much each time it passes a fiber/water transition, and can enter deeper into the fabric before it is eventually directed out of the fabric. The path traveled by the light in this case is longer, on average, than with dry fabric. Much of the light can actually pass the fabric if you didn't pick your swimwear right. :-) If there are light-absorbing impurities in the fabric (there always are some), such as dye molecules, then the longer path travelled by the light in the wet cloth results in higher absorption, hence deeper shade. -olli From: oniemita at mail.student.oulu.fi (Olli Niemitalo)
Date: Mon, 8 Sep 2003 08:54:31 +0300 (EEST)
Subject: Bleached (maybe PFD) cotton flannel from Dharma looks pink at a bunch but white in isolation, what is this?
Does this happen under the same lighting conditions? Under different lights, objects can appear a different color. This is both because of the different spectra of the lights and the adaptation of your eyes to accept the lighting as being white. When there are two different light sources (a window and a lamp, or two different types of lamps), an object might only be illuminated by either if the object surface is at a funny angle or if the object is shadowed from one of the light sources. Also, your eyes will somewhat compare the color of the object against the background against which you view it. Lastly, if you stare at some colored object for a while, your eye/brain will compensate against this perception even a short time after you have turned your eyes away, making other objects appear a different color. Apart from medical reasons, perhaps you can find an explanation from these? -olli From: o at iki.fi (Olli Niemitalo)
Date: Wed, 1 Oct 2003 10:18:54 +0300 (EEST)
Subject: In Dye Mixer, which Procion MX are used? I have two suppliers that sell somewhat different shades with the same name.
Most of the Procion MX dyes in the Dye Mixer software came from Quilt und Art in Germany. I have presumed that pure dyes with the same (Procion MX) name may only vary by concentration and by additive content. If this is the case (and I hope it is!), you should be able to get near-identical results by adjusting the amount you use, until the actual amounts of dye chemical match. Pre-mixed dyes can be different as dye houses do their own mixes. Some mixed dyes are also included in the Dye Mixer. For a chart of pure Procion MX dyes, see Paula's page: http://www.pburch.net/dyeing/FAQ/pureMXcolors.shtml -olli From: o at iki.fi (Olli Niemitalo)
Date: Sun, 19 Oct 2003 22:10:30 +0300 (EEST)
Context: I tried the Dye Mixer applet, but I get different colors using my real-life recipes.
As you guessed, this has to do with the dye Amount numbers. They are not
directly the real-life dye amounts ("how much dye should I use"), but they
are proportional to those. Let me explain: If you use 1 g of dye A and 1 g
of dye B, you may get consistent results using the same fabric, bath
volume, temperature, stirring or no stirring, time, pH, salt/urea
concentration, etc.. But as soon as you change these parameters,
you may get different results. Dye Mixer is not "locked" into a specific
set of parameters, as different people have different ways of doing
things and I didn't want to be biased.
So, I can only suggest that you find by experimenting the
dye-specific ratios between the Amount values and the real-life dye
amounts. For example, if you see that 2 g of a certain dye gives the same
color as Amount = 0.5, then you know that one Amount unit corresponds
to 4 g of the dye (calculated as 2 g / 0.5). The ratio is different
for each dye!
Please let me know whether you succeed in reproducing your mixes after
this calibration step!
Ultimately, it would be best if Dye Mixer had some useful presets, or
a semi-automatic calibration procedure, but I cannot promise this.
Some good news for you, Carol: Both Yellow MX-GR and Blue MX-R, and plenty
of other MX dyes, will be added to Dye Mixer very soon (within next
week?).
-olli
From: o at iki.fi (Olli Niemitalo)
Date: Sun, 7 Mar 2004 20:14:36 +0200 (EET)
Context: Dye Mixer inaccessible. I changed my DyersLIST e-mail address, is this the reason?
It is probably a temporary server problem. The following address is the one you should be using: http://www.iki.fi/o/dye/dyemixer/ Some you might be waiting for updates to the software that I promised to make. Unfortunately, I have been very busy with studies, at the cost of no time left for the project. Hopefully this will change in a couple of months. In case of "emergency", try this link for another server: http://www.ee.oulu.fi/~ollinie/dye/dyeresize.html No, the software is not associated with Dyer's List. -olli From: o at iki.fi (Olli Niemitalo)
Date: Thu, 16 Aug 2007 00:43:00 +0300
Context: Discharging Procion MX from cotton
There's a table here:
http://yehar.com/bleach.txt It's from an old DyersLIST e-mail: https://list.emich.edu/mailman/private/dyerslist/2003-June/019070.html -olli From: o at iki.fi (Olli Niemitalo)
Date: Tue, 27 Jan 2009 13:23:57 +0200
Context: How can I sprinkle Procion MX powder without lumps for snow dyeing?
In the kitchen, one can sprinkle flour through a sift to avoid clumps. Could this work for dye powder? -olli From: o at iki.fi (Olli Niemitalo)
Date: Wed, 28 Jan 2009 10:06:54 +0200
Context: What do we really know about reactive dye safety?
I did a search on PubMed and the best article that came up was this Croatian study: Zuskin E, Mustajbegovic J, Schachter EN, Doko-Jelinic J. Respiratory function of textile workers employed in dyeing cotton and wool fibers. Am J Ind Med. 1997 Mar;31(3):344-52. >From the abstract: "Our data suggest that textile dyeing workers develop acute and chronic respiratory impairment as a result of their exposures. These findings are exacerbated by cigarette smoking." http://www.ncbi.nlm.nih.gov/pubmed/9055958 In the article they cite previous findings from literature: (some citations removed and emphasis added) "There are numerous publications on the effect of dust on the respiratory system of textile workers employed in processing textile fibers such as cotton, hemp, flax, and wool. However, there are few available data on respiratory function in the workers employed in the textile dyeing industry. Work-related respiratory symptoms among employees in wool dye-houses in the United Kingdom associated with exposure to Lanasol dyes were reported by Topping et al. [1989]. Viegi et al. [1985] evaluated respiratory function in workers of a dye factory and found the prevalence of chronic bronchitis and dyspnea of 32%; flow rates were significantly lower than reference values. Among their workers, 71% had diagnoses of chronic obstructive lung disease; they all worked >15 years in the dye textile industry. Nine cases of immediate type occupational asthma due to reactive dyes in one dye industry were described by Park et al. [1989]. Docker et al. [1987] showed that >15% OF WORKERS HANDLING REACTIVE DYES HAD WORK-RELATED RESPIRATORY OR NASAL SYMPTOMS. The same authors considered that the symptoms could be attributed to an irritant response to chemicals used in this industry, including hydrochloric acid vapor, sulfur dioxide, as well as the reactive dyes themselves. SOME OF THE SYMPTOMS WERE ATTRIBUTED TO AN ALLERGIC REACTION TO SPECIFIC AGENTS IN THE REACTIVE DYES. Recently, Lin et al. [1995] found that reactive dye workers with atopy or asthma were at a significantly higher risk of developing respiratory symptoms. Alanko et al. [1978] reported four cases of immediate-type occupational allergy (asthma and rhinitis) to reactive dyes. According to these authors, reactive dyes probably act as haptens. Interestingly, Park et al. [1990] described that reactive dye induced occupational asthma without nonspecific bronchial hyperreactivity." My comment is that as each dye is a different chemical, and there is also simultaneous exposure to other chemicals, it is hard to tell which one is causing the symptoms of the workers. There *are* allergy risks associated with *some* dyes. It is better to play safe with all of them if you don't know which. Inhalation is how one gets into worst trouble, but also skin sensitization is a possibility. I have not seen anything about ingestion. There is another article from the same authors on wool dust: Zuskin E, Mustajbegovic J, Schachter EN, Kanceljak B, Godnic-Cvar J, Sitar-Srebocan V. Respiratory symptoms and lung function in wool textile workers. Am J Ind Med. 1995 Jun;27(6):845-57. http://www.ncbi.nlm.nih.gov/pubmed/7645578 >From the abstract: "Our data suggest that dust exposures in wool textile mills may be associated with the development of chronic respiratory symptoms and impaired lung function." -olli
From: o at iki.fi (Olli Niemitalo)
Date: Mon, 16 Feb 2009 16:49:25 +0200
Subject: Ultrasound cleaners
Has anyone tried ultrasound cleaners ("sonicators") in dyeing?
I'm reading an article that says that ultrasound vibration improves
dye penetration and dye uptake in many wet dyeing processes (Vajnhandl
S and Le Marechal AM, Ultrasound in textile dyeing and the
decolouration/mineralization of textile dyes, Dyes and Pigments,
Volume 65, Issue 2, May 2005, Pages 89-101).
-olli
From: o at iki.fi (Olli Niemitalo)
Date: Mon, 3 Aug 2009 12:36:19 +0300
Context: Organic PFD fabric
In its Global Organic Textile Standard, GOTS allows up to 10 % of synthetic (polyester, polyurethane, polyamide) or regenerated (viscose, acetate, Lyocell) fibers, or up to 25 % in case of socks, leggings and sportswear. -olli From: o at iki.fi (Olli Niemitalo)
Date: Mon, 3 Aug 2009 12:44:04 +0300
Context: Organic PFD fabric
On first reading I missed that in the following case the item may be labeled MADE WITH X% OF ORGANIC MATERIALS. To be labeled ORGANIC, the limit is more strict, 5 %. -olli From: o at iki.fi (Olli Niemitalo)
Date: Thu, 6 Aug 2009 10:54:35 +0300
Context: Can thiox be used to discharge jeans?
Sodium dithionite did not remove indigo from my jeans. The same is probably true for thiox as another reducing agent. -olli From: o at iki.fi (Olli Niemitalo)
Date: Sat, 9 Jan 2010 12:19:31 +0200
Subject: Who should be the new list moderator?
I greatly valued Pat's to-the-point way of running the list. Whenever she had to interfere, she did it in a neutral and objective manner, gaining everyone's respect. I can easily see Paula as one person to continue the tradition. -olli From: o at iki.fi (Olli Niemitalo)
Date: Sun, 27 May 2012 09:47:46 +0300
Subject: How can I create a gradient from one color to an entirely different color?
To guarantee a monotonic gradient: Hang the piece from one end and dip the other end quite deep into the dye bath, lift up completely and add more dye into the bath, dip again but this time a little less deep. Repeat lifting, adding dye, and dipping until you have the complete gradient for one color. Wash the piece to remove free dye and flip the piece upside down. Repeat the complete process for another dye gradient that starts from the opposite end. -olli From: olli.niemitalo at gmail.com (Olli Niemitalo)
Date: Sun, 3 Jun 2012 14:10:54 +0300
Subject: Making list archives web-searchable
Hi all, I propose to make the DyersList archives web-searchable. The current archives would be sent to mail-archive.com and they would put them visible on the web. A "bot" account of mail-archive.com would also be subscribed to the list so that new messages would get stored and made visible immediately. You can read more about the process here: http://www.mail-archive.com/faq.html#newlist Then, if anyone does a web search on subjects discussed on the list, the relevant postings will likely appear in the search results. The motivation is that there is much valuable information on the list and it will benefit more people if it is more easily searchable and accessible. The number of frequently asked questions would also decrease as the answer might be found by a web search. Currently the list archives are accessible to anyone who joins the list, and anyone can join, so in that way the archives have always been public. During 1997-2001 the archives were web-searchable, but this changed as a new list search system was put in place. I don't think that the current importance of web searches was foreseen at the time. I'm willing to take risk that a change in the visibility level of the archives and on their technical implementation is covered by the mailing-list implied license on the use of the postings which are the property of their authors. The list guidelines instructs to keep private messages off the list, so there should not be a problem there either. I tried to contact Brooks Stevens at East Michigan University to hear if they have a say on the matter, but did not get a reply. If there is no opposition to the development, I will go forward with this. If you do not want your past postings to appear in web-searchable archives, please let me know the associated e-mail addresses and I will delete the postings from the web-searchable archives before they go on-line. -olli From: Olli Niemitalo <o@iki.fi>
Date: Mon, 4 Jun 2012 10:39:10 +0300
Subject: Re: Making list archives web-searchable
EMU has said "no" to this. So it is not going to happen. I will not discuss the matter further, on-list. -olli